The Ou Lab
Studying early RGC degeneration leading to glaucoma
Our Research
Our laboratory studies the cellular and synaptic mechanisms of glaucomatous neurodegeneration. Our research program combines imaging and analysis of specific cell and synaptic labels in the retina, rodent models of experimental glaucoma, novel genetic tools in which specific cell types are labeled, and the tools of molecular/cell biology and physiology to address a series of questions that, unanswered, have prevented progress in the field: 1) What are the early steps of compartmentalized neurodegeneration of the ganglion cell in glaucoma? 2) Are there specific ganglion cell types that are more susceptible to intraocular pressure (IOP) elevation? 3) Are there specific inner retinal circuits that are more susceptible to IOP elevation? As a clinician-scientist, I am motivated to advance our field not only by answering these fundamental questions, but also by translating the knowledge gained into improvements in diagnostic and treatment modalities in glaucoma. For example, a detailed understanding of the earliest structural and functional changes that occur in glaucoma will allow us to design treatments that can rescue RGCs, perhaps the most susceptible RGCs, before irreversible cell death occurs. Our long-term goal is to understand the mechanisms underlying ganglion cell degeneration and circuit remodeling in glaucoma in order to improve diagnosis and treatment.
-
What are the early steps of compartmentalized neurodegeneration of the ganglion cell in glaucoma?
A major gap in our treatment paradigm for glaucoma is that IOP lowering treatment is often initiated after there is already evidence of RGC death and visual field loss. A barrier to improving treatments is that our understanding of the pathological cellular processes that occur between initial axonal injury and eventual apoptotic soma death is lacking. Growing evidence supports the hypothesis that the pathological processes an RGC undergoes when stressed may be compartmentalized at the subcellular level, e.g. the soma, axon, dendrite, or synapse. We use a laser-induced ocular hypertension model in mice to study early structural alterations at the level of the dendrite and synapse in ganglion cells.
Our recent work demonstrates that synapse loss is one of the earliest, and perhaps first, structural alterations in ganglion cell degeneration. We found that synaptic pruning is followed by loss of dendritic area and complexity. Our findings support the hypothesis that there is a critical window during which synapses are pruned, but major structural alterations have yet to occur, a window that may be a promising target for novel therapies.
-
Are there specific ganglion cell types that are more susceptible to IOP elevation?
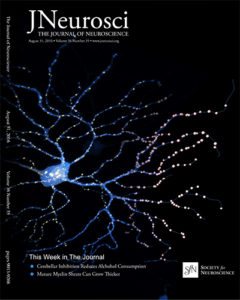 A longstanding question in glaucoma pathophysiology is the varying susceptibility of the ganglion cells and the optic nerve itself to injury. In collaboration with Luca Della Santina (University of Pisa) and Rachel Wong (University of Washington), we used biolistic transfection of individual RGCs and multielectrode array recordings to examine the effects of transient IOP elevation on the structure and function of specific RGC types. Our results reveal type-specific differences in RGC responses to injury with a selective vulnerability of OFF-transient alpha-type RGCs, and furthermore, an increased susceptibility of synapses in the OFF sublamina. The “selective vulnerability” of specific RGC types offers new avenues for the design of more sensitive functional tests and targeted neuroprotection.
A longstanding question in glaucoma pathophysiology is the varying susceptibility of the ganglion cells and the optic nerve itself to injury. In collaboration with Luca Della Santina (University of Pisa) and Rachel Wong (University of Washington), we used biolistic transfection of individual RGCs and multielectrode array recordings to examine the effects of transient IOP elevation on the structure and function of specific RGC types. Our results reveal type-specific differences in RGC responses to injury with a selective vulnerability of OFF-transient alpha-type RGCs, and furthermore, an increased susceptibility of synapses in the OFF sublamina. The “selective vulnerability” of specific RGC types offers new avenues for the design of more sensitive functional tests and targeted neuroprotection.
-
Are there specific inner retinal circuits that are more susceptible to IOP elevation?
Given the finding that OFF RGC types and synapses in the OFF sublamina of the IPL are more vulnerable to IOP elevation, our hypothesis is that cells stratifying in the OFF sublamina are more vulnerable to IOP elevation, and that the OFF circuit may be damaged earlier and to a greater extent than the ON circuit. While there has been detailed analysis of the cellular alterations to ganglion cells in glaucoma, little attention has been paid towards the presynaptic partner of the ganglion cell, the bipolar cell. A lack of understanding of the effects of elevated IOP on the bipolar cell to ganglion cell synapse is a critical barrier towards detailed knowledge of how the inner retinal circuitry is dismantled in glaucoma. Our overall objective is to determine if, when, and how RGC-bipolar cell connections and bipolar cells are altered after IOP elevation. There is an urgent need to understand how this circuitry is dismantled and potentially rearranged in order to be successful at developing diagnostics that target susceptible pathways and therapeutic strategies such as regeneration of injured RGCs that require intact inner retina circuitry.