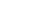Research
“Live the questions now. Perhaps then, someday…you will gradually, without even noticing it, live your way into the answer.” ~ Rainer Maria Rilke
Central Questions
The retinal pigment epithelium (RPE) is both the guardian and the Achilles’ heel of the retina: it performs numerous functions that are indispensable for the health of the photoreceptors and for vision; but damage to the RPE is also the initiating factor that eventually leads to vision loss in macular degenerations. Aging in the RPE is characterized by the accumulation of vitamin A metabolites (lipofuscin) in RPE lysosomes and lipid-protein aggregates (drusen) above and beneath the RPE. Whether these deposits are a cause or consequence of RPE dysfunction is unclear. Currently, there are no approved treatments for either inherited macular degenerations or the most prevalent for of age-related macular degeneration (called dry AMD or geographic atrophy). This lack of therapeutic success is largely due to limited insight into basic RPE biology, and how this is altered in aging and disease.
Our lab’s current research is focused on three broad areas:
- How is RPE homeostasis maintained over a lifetime?
- What is the tipping point that drives the retina away from normal aging towards AMD?
- How can we apply this information to design effective therapies?
To address these questions, we use high-resolution, high-speed, and super-resolution imaging to investigate critical pathways that maintain RPE homeostasis. Our lab has pioneered the use of high-speed live-cell imaging of polarized, differentiated RPE monolayers: we used advanced live imaging to establish how autophagosome biogenesis and trafficking are impaired in models of macular degeneration (Toops et al., 2015). We were the first to use live-cell total internal reflectance fluorescence (TIRF) microscopy to investigate how the RPE maintains membrane integrity after complement attack (Tan et al., 2016). More recently, we captured newborn endosomes as they form from the RPE plasma membrane with exquisite temporal and spatial detail (Kaur et al., 2018).
By carefully dissecting mechanisms that regulate essential RPE functions, we have been successful in weaving together disparate cellular pathways to provide a coherent image of events that could contribute to macular degeneration. Key concepts we have recently elucidated include:
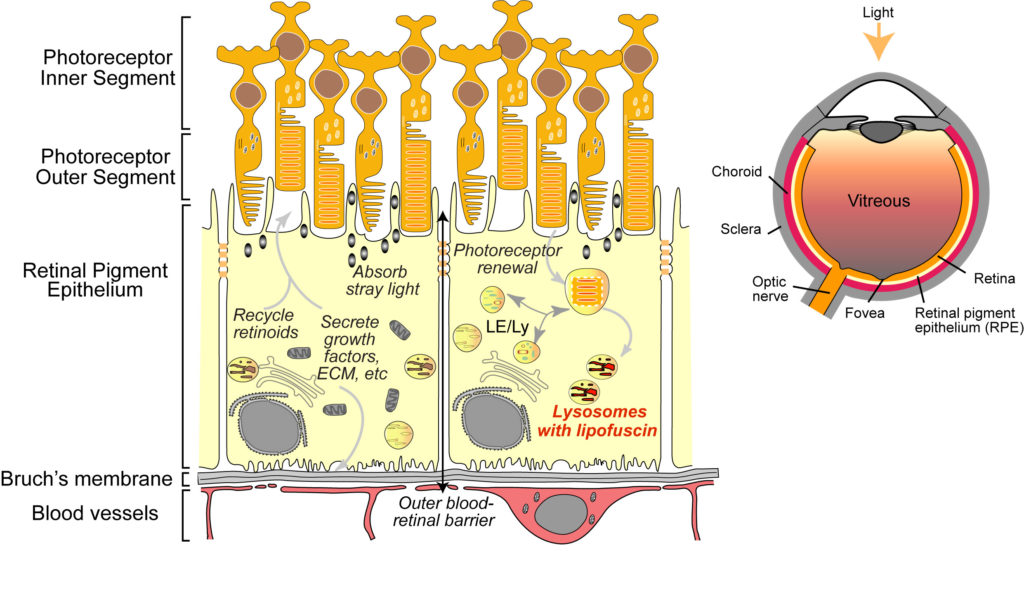
1. Autophagic defects are an early sign of macular degeneration.
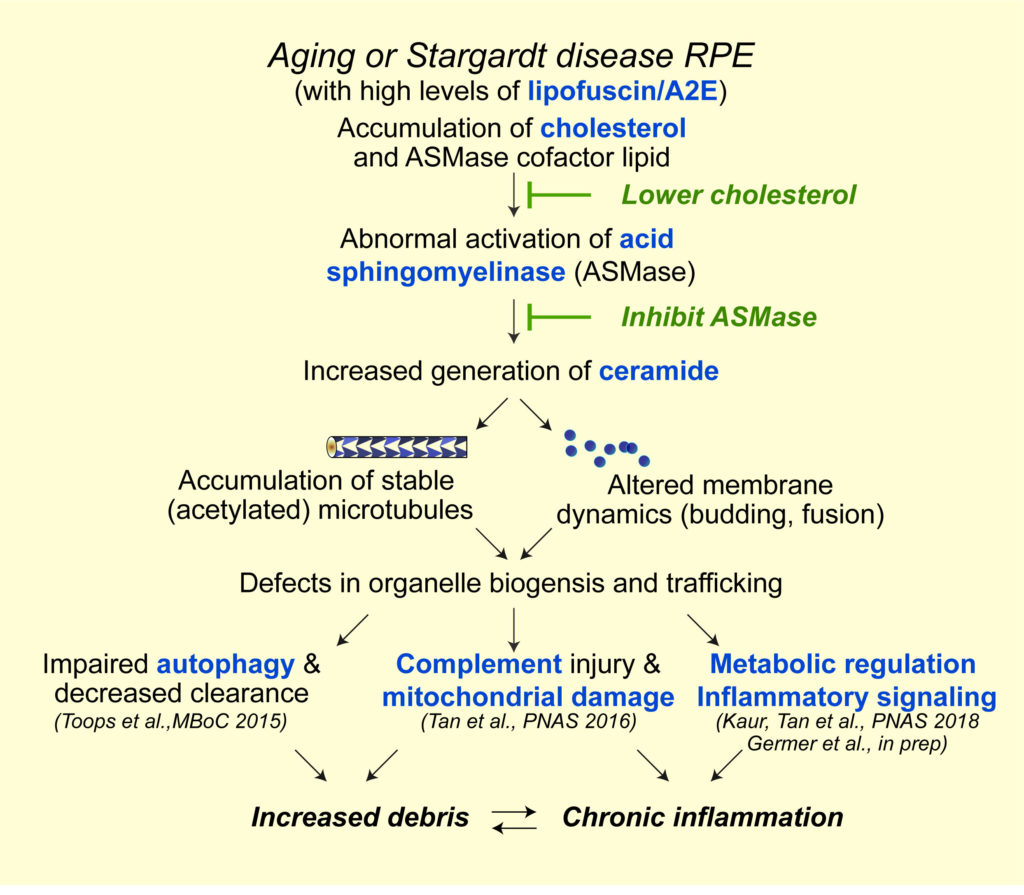
Autophagic defects contribute to neurodegenerative diseases; however, its role in the pathogenesis of retinal dystrophies has been unclear. Using live-cell imaging of primary adult RPE monolayers and the Abca4-/- Stargardt disease mouse to model early macular degeneration, we were the first to identify age-related defects in autophagy in the RPE (Toops et al., Mol Biol Cell, 2015). We showed that excess cholesterol in RPE lysosomes due to lipofuscin bisretinoids (Lakkaraju et al., PNAS 2007) activates acid sphingomyelinase (ASMase), which increases the accumulation of acetylated microtubules. Impaired microtubule-based transport interferes with autophagosome biogenesis and trafficking, and autophagic flux.
Publications:
- Toops KA, Tan LX, Jiang Z, Radu RA and Lakkaraju A. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Molecular Biology of the Cell. 26: 1-14, 2015. PMCID: PMC4279221.
- Toops KA, Tan LX and Lakkaraju A. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Experimental Eye Research, 124C: 74-85, 2014. PMID: 24861273
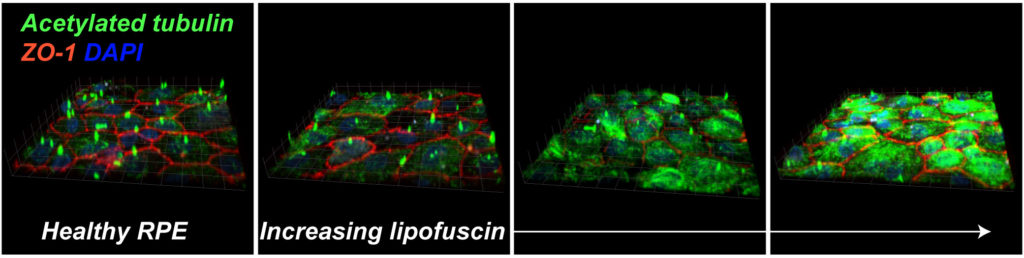
2. Two rapid-response mechanisms help the RPE combat complement-mediated inflammation.
Abnormal activation of the complement system is a hallmark of macular degenerations, but how does the RPE protect itself from complement? Our lab was the first to identify two critical mechanisms – rapid recycling of the complement-regulatory protein CD59 and membrane repair by lysosome exocytosis –that limit complement-induced RPE damage (Tan et al., PNAS, 2016). These studies build on our earlier work where we identified the molecular machinery required for polarized lysosome exocytosis in epithelial monolayers (Xu et al., J Cell Sci, 2012). In models of macular degeneration, cholesterol-mediated activation of ASMase derails organelle traffic and inhibits both protective responses, leading to mitochondrial fragmentation after complement attack. Importantly, this research identified a molecular link between cholesterol and complement, two pathways that have long been implicated in AMD.
Publications:
- Tan LX, Toops KA and Lakkaraju A. Protective responses to sub-lytic complement in the retinal pigment epithelium. Proceedings of the National Academy of Sciences USA 113: 8789-94, 2016. PMCID: PMC4978296.
- Xu J, Toops KA, Diaz F, Carvajal-Gonzalez JM, Gravotta D, Mazzoni FM, Schreiner R, Rodriguez-Boulan E and Lakkaraju A. Mechanism of lysosome exocytosis in polarized epithelial cells. Journal of Cell Science 125: 5937-5943, 2012. PMCID: PMC3585513
3. Early endosomes act as conduits for intracellular complement activation and metabolic reprogramming in the RPE.
Using high-resolution live-cell imaging of human donor tissue and mouse models, we show that age-related and pathological accumulation of vitamin metabolites increases ceramide at the apical surface of the RPE, which promotes inward budding and homotypic fusion of early endosomes. These enlarged endosomes internalize the complement protein C3 into the RPE, and facilitate the intracellular generation of C3a fragments that regulate critical metabolic processes such as activation of the mechanistic target of rapamycin (mTOR). Our data link aberrant early endosome biogenesis with complement activation and metabolic reprogramming in the retina, and support therapeutic targeting of ceramide in macular degenerations and other neurodegenerative diseases.
Publication:
- Kaur G*, Tan LX*, Rathanasamy G, La Cunza N, Germer CJ, Toops KA, Fernandes M, Blenkinsop TA and Lakkaraju A. Aberrant early endosome biogenesis mediates complement activation in models of macular degeneration. Proceedings of the National Academy of Sciences USA 115: 9014-19, 2018. PMID: 30126999.
4. Clinically approved drugs can be repositioned to treat macular degenerations
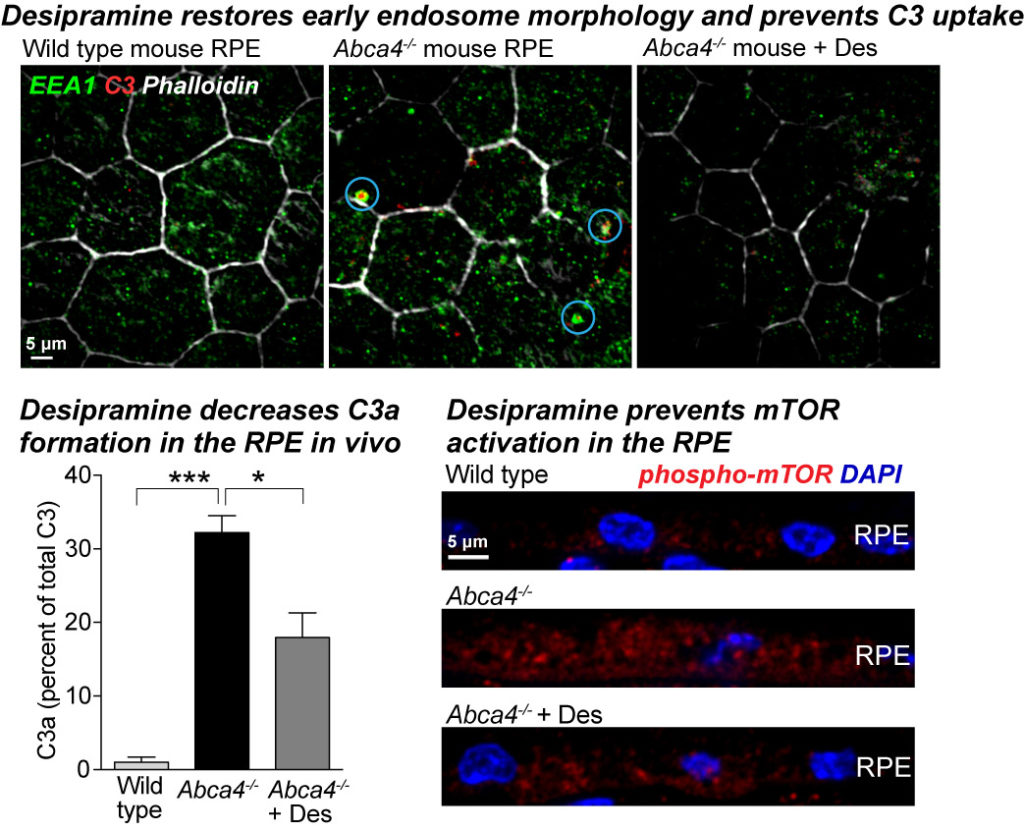
As a direct extension of the above research, we identified two drug targets – decreasing cholesterol or inhibiting ASMase. Clinically approved drugs that are known to inhibit ASMase as promising therapeutics for macular degenerations. Our studies show that ASMase inhibition not only improves autophagy and lysosome function but also decreases complement-mediated mitochondrial injury and metabolic dysfunction in Abca4-/- mice. We recently reported that the antidepressant desipramine, which lowers ceramide levels by inhibiting acid sphingomyelinase, corrects early endosomal defects in the RPE of Abca4-/- mice. This prevents C3 internalization, limits the formation of C3a fragments within the RPE, and prevents sustained mTOR activation (Kaur et al., PNAS 2018).
These approved drugs have documented safety and efficacy profiles; they are orally bioavailable, precluding the need for invasive delivery methods like intravitreal injections, and the use of ASMase inhibitors in humans is associated with a significantly decreased risk of developing AMD. We are now evaluating small molecule drugs that lower RPE ceramide in preclinical models of macular degeneration.
Publications:
- Kaur G*, Tan LX*, Rathanasamy G, La Cunza N, Germer CJ, Toops KA, Fernandes M, Blenkinsop TA and Lakkaraju A. Aberrant early endosome biogenesis mediates complement activation in models of macular degeneration. Proceedings of the National Academy of Sciences USA 115: 9014-19, 2018. PMID: 30126999.
- Tan LX, Toops KA and Lakkaraju A. Protective responses to sub-lytic complement in the retinal pigment epithelium. Proceedings of the National Academy of Sciences USA 113: 8789-94, 2016. PMCID: PMC4978296.
- Lakkaraju A, Toops KA, Tan LX. Use of Inhibitors of Acid Sphingomyelinase to Treat Acquired and Inherited Retinal Degenerations. U.S. Patent 14/746221