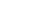The Lamba Lab
Focusing on retinal repair and modeling disease with stem cells technologies
Our Research
The Lamba Lab’s research focuses on the retina which is the main light-sensing region of the eye. The retina is subject to a variety of inherited and acquired degenerative conditions which lead to blindness due to loss of the light-sensing cells called photoreceptors. Like other regions of the CNS, loss of photoreceptors in the mammalian retina is not associated with any effective regeneration from resident cells. During his PhD and post-doctoral training, Dr. Lamba worked on developing and perfecting an approach towards pluripotent stem cell-based therapy for retinal degenerations due to photoreceptor cell loss (Lamba et al., PNAS 2006, Lamba et al., Cell Stem Cell 2009).
The Lab have been working on two broad areas: (a) feasibility and hurdles to photoreceptor replacement therapy and (b) modeling retinal degenerations in vitro.
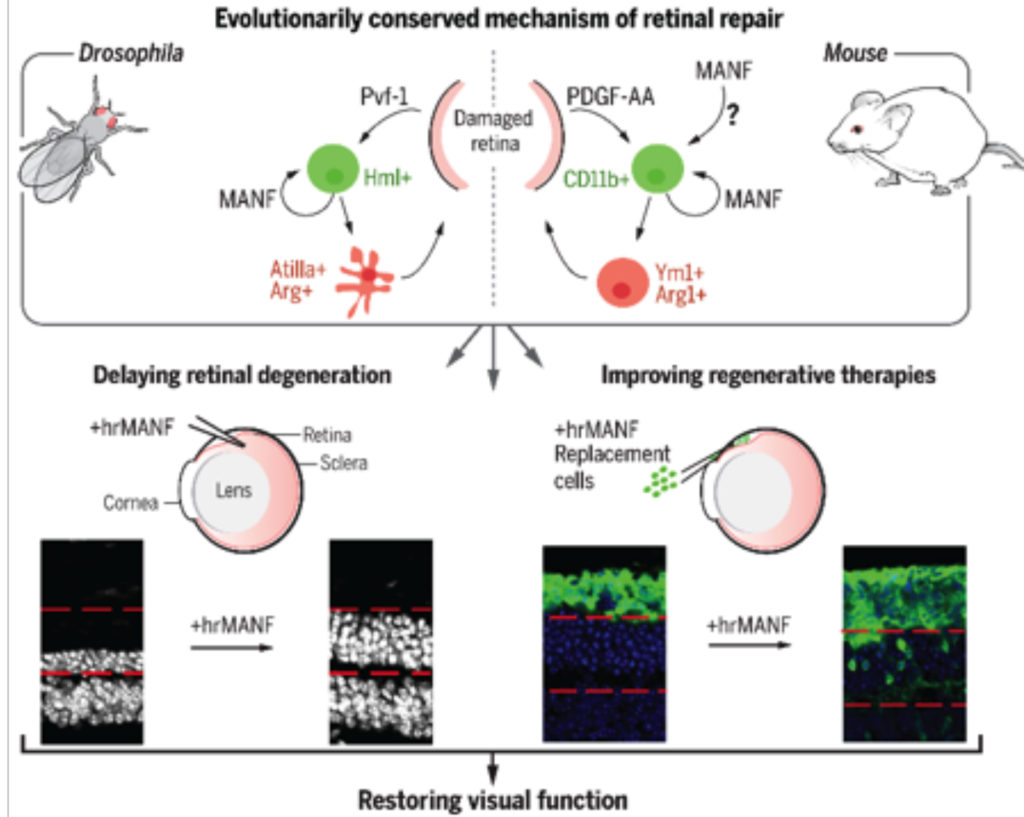
Barriers to cellular integration
We and others have demonstrated morphological and functional integration of primary mouse photoreceptors, as well as hESC-derived retinal photoreceptors into the retinae of mouse models of retinal degeneration. However, the efficiency of integration is low, with transplanted cells representing less than one percent of all cells in the host retina. Enhancing integration capacity is thus critical towards development of successful therapies for vision loss. One of the key projects in the lab was to identify immune-modulatory factors that can promote a pro-repair retinal microenvironment which can contribute to regenerative success. Working in collaboration with Dr. Jasper’s lab which has expertise in Drosophila repair mechanisms, we identified and characterized an evolutionarily conserved immune-cell secreted immune-modulatory factor, MANF (Mesencephalic Astrocyte-derived Neurotrophic Factor). We show that MANF promotes alternative activation of the microglia and macrophages leading to a pro-repair phenotype in flies and in mice. Exogenous supplementation of recombinant MANF was neuroprotective in multiple mice models of retinal degeneration. More importantly, MANF supplementation promoted integration of exogenous mouse photoreceptors and improved functional integration in a degenerative host environment. This report was recently published in Science (Neves et al. Science 2016).
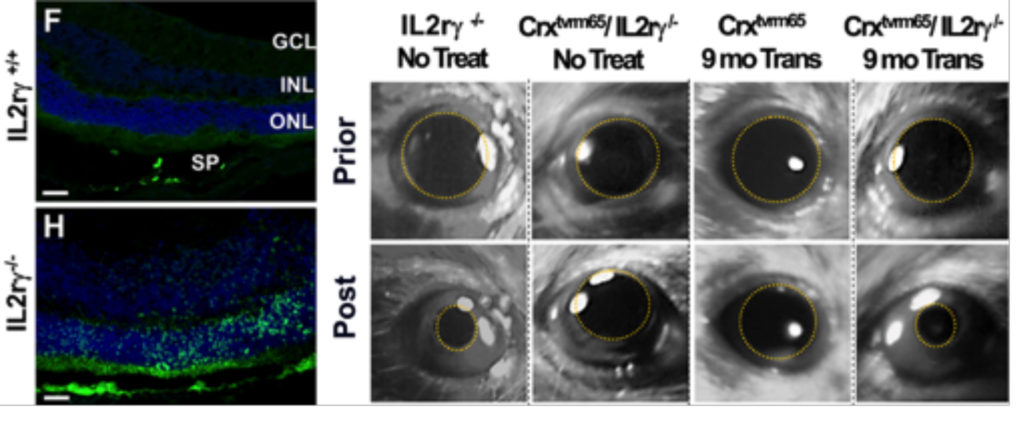
We have another project looking into cell rejection as a barrier to successful photoreceptor integration. Though the retina has classically been considered immune-privileged, recent reports suggest that this privilege may only be relative and get further compromised following retinal degeneration-associated inflammation and remodeling. Using mice lacking natural killer cells which are involved typically in transplant rejection, we show that human photoreceptor integration capacity can be significantly improved. This has also allowed us to now look at long-term functional integration. We observed restoration of light sensitivity in congenitally blind mice as far out as 9-months post injection. This report was recently published in Cell Stem Cell (Zhu et al. Cell Stem Cell 2016).
Future Directions: We are continuing our efforts to identify and break various critical barriers to effective integration of transplanted cells.
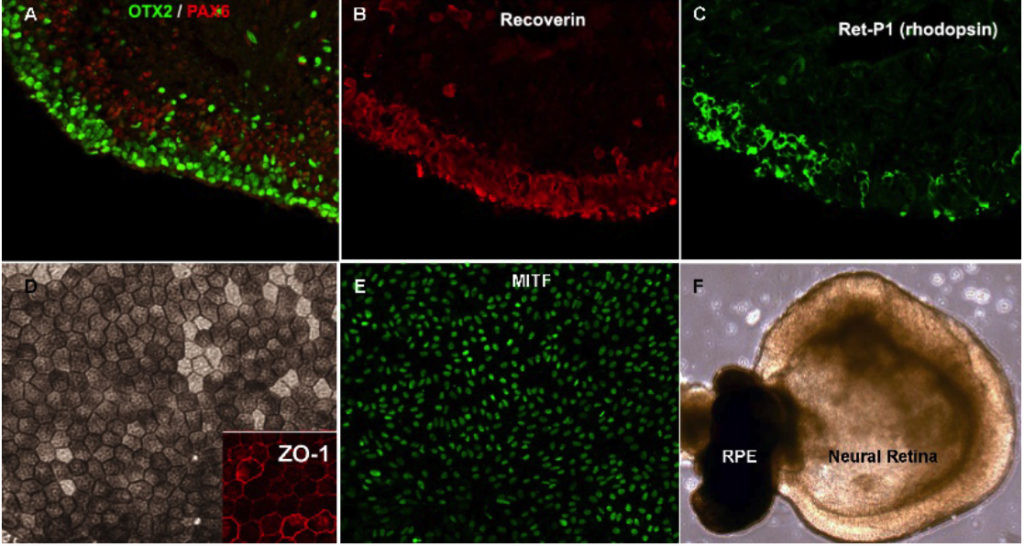
Disease Modeling in vitro and Drug Discovery Platform
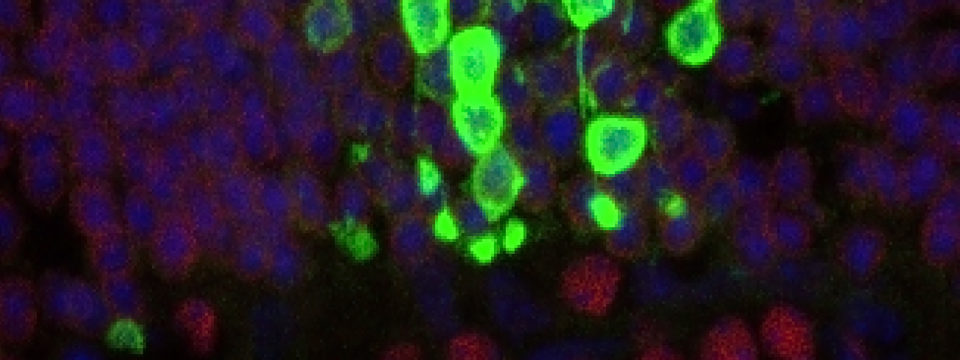
One of the other major efforts in the lab has been to use human pluripotent stem cell-derived retinal tissue for modeling various aspects of diseases in vitro. Our initial efforts have been focused on Age-related macular degeneration (AMD). AMD is a multifactorial polygenic disease closely associated with chronic environmental stress and aging for which there are no effective treatments especially for the more common dry-form of the disease. One of the projects in the lab was to develop an in vitro chronic stress model using human pluripotent stem cells-derived retinal tissue to gain insight into stress signaling over weeks of oxidative stress. In the resulting manuscript, we show that the human stem cell system had distinct advantages over immortalized lines which have previously been used for such studies. We also identified dynamic changes in the effector response of the NRF2 signaling pathways and microRNA changes associated with it. Finally, we identified a small molecule partial-agonist of the pathway which was protective against oxidative stress damage (Garcia et al. IOVS 2015).
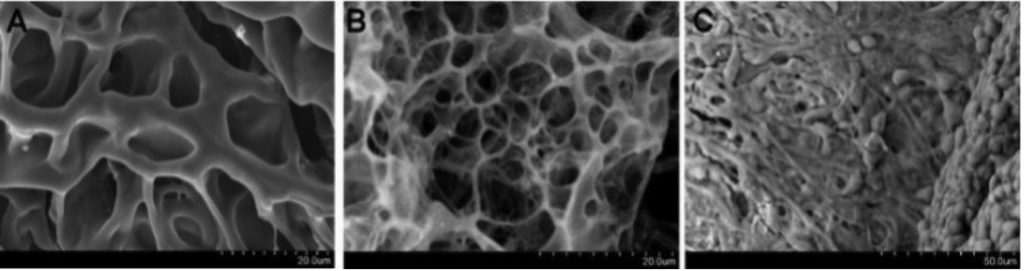
Future Directions: We are further continuing our efforts in disease modeling. We have ongoing projects using bioengineered scaffolds as well as new in-lab 3D printing technologies using custom generated hydrogel bioinks. We plan to use these approaches to look at inter-tissue interactions between the various layers in the eye including neural retina-RPE interactions and retina microglial/macrophage interactions. These combined with iPSC technologies to test cross-pathogenesis under diseased conditions will be a critical tool towards better disease models and provide better educated approaches towards therapies. Towards this, we are already working on proteomic and secretomic characterization of iPSC-retina following stress.
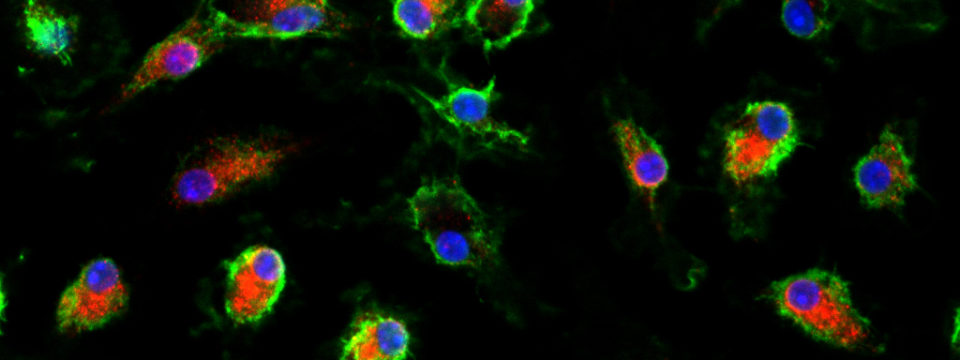
In addition, we have embarked on newer projects incorporating 3D retinal organoid systems. We are combining them with genome editing technologies using CRISPR/Cas9 to understand retinal development and create efficient 3D models for disease pathogenesis studies. Starting out with mouse embryonic stem cell-derived retina, we plan to move these technologies to human retinal 3D tissues as well to generate SNP-specific disease models.